Fibre
Chemically speaking, wool is keratin, a protein copolymer containing about 17 different amino-acid monomers. The main elements are cystine, leucine, glycine and glutamic acid. Covalent cross-linking of adjacent cystine residues by disulphide bonds is a major factor for the mechanical properties of keratin fibres. The bond fragility to high temperature (over 90-100°C), alkaline pH, reduction or oxidation, must be taken into account in dyeing.
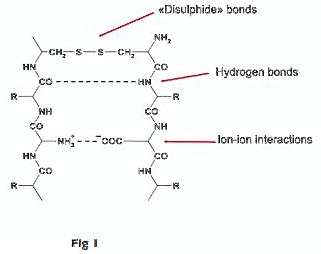
The rather strong disulphide bonds (-S-S-) are supplemented by weaker hydrogen bonds between -NH- and -CO- groups of adjacent keratin chains, ion-ion interactions between amino groups (in their protonized cationic form) and carboxylic acid groups (in their anionic form) which are part of the keratin macromolecules, and hydrophobic bonds between adjacent hydrophobic aliphatic chains.
The unusual elasticity of the wool fibre in its relaxed form is explained by keratin's natural folded state.
Like most natural fibres, wool has a heterogeneous structure. Its main characteristics include a hydrophobic outer cuticle (scales) and strong, highly oriented fibrous bundles embedded in amorphous protein remnants.
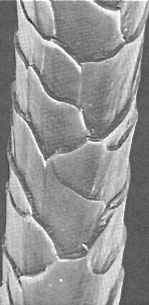
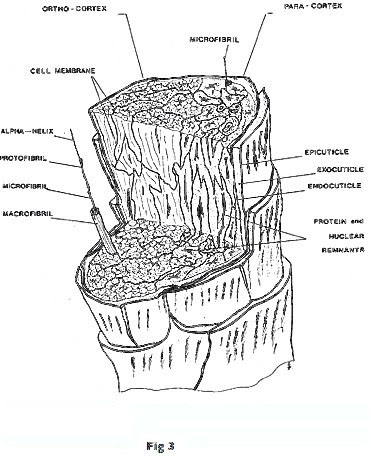
The quality of wool, its textile and dyeing properties are determined by fibre fineness, length, scale structure, natural shade, brightness, cleanliness and freedom from damage. It depends on many factors, mainly sheep breed, age, health and stress of the sheep, season of shearing, body part it comes from (finest comes from shoulders, coarsest from breech), the climate it was exposed to, food, the thoroughness of fibre scouring (full or part removal of lanolin) etc. Central Asian nomads distinguish and have specific names for dozens of wool qualities. In general, they prefer semi-fine and coarse (25-35 microns) spring wool for carpet weaving. The degree of scale abrasion, (due to weathering, to sheep peregrinations through shrubs, to a modern chemical treatment like chlorination, or to an antique one like wool fermentation) has a large impact on wool dye-ability (in particular on levelness, depth and reproducibility of shade), on its tendency for “felting”, on its softness and brightness, etc.
The pile wool of many classical Ushak rugs shows a significant scale opening and exfoliation, probably caused by 8-15 days cold fermentation of the wool under mildly acidic pH in the presence of some alum, before actual mordant dyeing. (Sources: Dr. Manfred Bieber, 7. ICOC, June 1993 and recently confirmed by my friend, Marc Roy, a carpet weaver and expert user of natural dyes.)
Scales have a more hydrophobic and more compact (oriented) structure than the inside of the wool fibre and therefore create a barrier to dye penetration into the fibre, retarding its diffusion. Scales also differentiate between dyes, favouring absorption of the most hydrophobic and small ones, slowing down and decreasing the uptake of the most hydrophilic and bulky ones.
Dyes and dyeing methods
Wool fibre heterogeneity makes level dyeing quite difficult to achieve. This explains why dyeing “in piece” (after a textile is woven) or as a yarn (for example, wool used for the pile of hand woven rugs) seldom result in dyeing evenly. Wool is mostly dyed in form of loose fibre packages (so called “loose stock” ), in which case several batches are carefully blended after dyeing and before yarn spinning, thus minimizing the effect of any poor levelness. Colour differences on fibres (some call them micro-abrash) are masked by this blending. Piece- and yarn dyeing are only performed with the best diffusing (levelling) types of dyes. Good levelness requires good dye diffusion, which means "easy in" but, unfortunately, also "easy out". As a consequence, piece- and yarn dyed wool (using rapidly diffusing dyes) has a lower wash- and wet fastness than loose stock-dyed wool (dyed with slowly diffusing dyes). Exceptions to this rule are when wool yarn is dyed with modern reactive dyes.
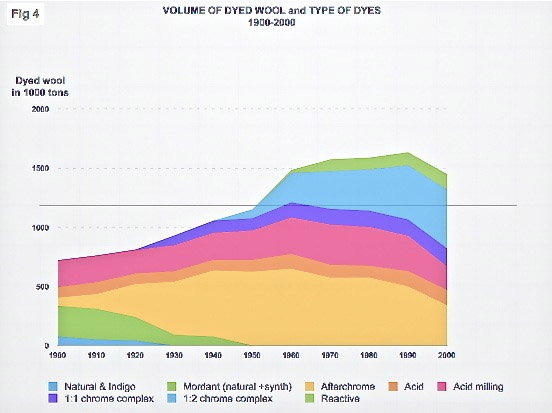
Since the invention of the first synthetic dyes, ten different types have been used for dyeing wool. Five of them are now fully obsolete, including four of the only five types ever used for dyeing the yarn for hand woven rugs.
The first synthetic dye is Saxon blue (1743, Barth), a natural indigo sulfonated with sulfuric acid (and, incidentally, a lousy dye for wool). It took another century to really get the eventful history of synthetic dyes started. The story of teenager Perkins inventing the phenazinium cationic dye, mauvein, in 1856 by mistake, and of Hoffmann and friends creating a whole new industry based on this invention, is well known by all carpetologists. Only a few of the early synthetic cationic triphenyl methane dyes (misnamed "aniline dyes"), were probably ever used to dye wool (a few bright magenta-, violet-, blue- and green dyes), but all had poor wet-fastness and disastrous light-fastness (rating 1, at best 1-2, of the 1 to 8 ratings on the so-called Blue Scale), because they turned beige or grey in the shortest time. It appears that they were wreaking havoc in some weavers workshops for at least five decades (which rather beats me!).
In 1858, Griess made a much more important discovery, triggering the explosive development of our modern dyestuff and pigment industry: the diazotisation of aromatic amines (such as anilines or naphtylamines) and their coupling with aromatic bases (such as phenols or naphthols), forming a so-called azo bridge (-N=N-). This creates large systems of conjugated double bonds and, therefore, strong chromophores (coloured molecules).
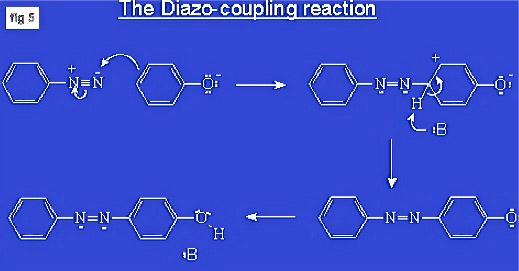
With dozens of suitable colourless building-blocks (aromatic amines and bases) to play with, chemists soon synthesized hundreds of strongly coloured combinations. Most of these new molecules included one or several sulfonic acid groups (-SO3H) making them soluble in water and leading to various new classes of dyes. The first was the so-called acid dyes, which were misused for dyeing rug yarn as early as 1865:
1. These too small and too hydrophilic molecules had insufficient affinity for wool and could not form stable metal complexes by mordanting, unlike the best red and yellow natural dyes. They soon became infamous for severe running (especially the di- and trisulfonated reds).
2. This problem was compounded by the poor light fastness of most red, scarlet and orange elements (2-3 or 3 on the Blue Scale).
Despite these serious handicaps, some carpet dyers continued to use Orange II, Ponceau 2R and 6R, Amaranth, etc., at least until 1930, attracted by their low cost and by their much brighter shades than natural mordant dyes (very bright blue acid dyes appeared later, around 1900, which explains why indigo kept its importance during several decades). Unfortunately, some carpet weavers used and abused this opportunity to make kitschy shades. In this case (and only in this case) a trained eye can tell that no natural dye was used. These acid dyes were the ancestors of modern levelling acid dyes.
In 1868 Graebe and Perkins developed industrial scale processes for synthetic alizarin (1,2 di-hydroxy anthraquinone), the main and best natural mordant dye in madder. Synthetic alizarin was a brilliant commercial success and quickly replaced madder in most wool markets (including wool for rugs), which it shared for the next 40 years with quite lousy, but cheaper and brighter acid reds.
Also around 1868, various chemists attempted to mimic natural mordant dyes. Their research took two main directions:
1. They sulfonated or aminated recently synthesized alizarin. Several quite light-and wet-fast wool red and blue dyes (Cr mordant) were marketed; for example, Alizarin Red S (Graebe 1871) and Alizarin Blue WX.
2. They used the newly invented azo chemistry as well as aromatic amines and bases carrying correctly positioned hydroxyl (-OH) and carboxylic acid (-COOH) groups, which together with the nucleophilic azo bridge would yield rather stable metal complexes when post-mordanted, mainly with chromium ions (for example Alizarin Yellow GGW).
A whole new class of synthetic acid mordant dyes, covering a broad range of shades (yellows, oranges and reds of rather subdued shades, a few rather clear blues, as well as browns, navies and blacks) was created. Judging from their chemical structure, most dyes were surely more costly than alizarin, indigo, or the acid dyes. I suspect that few of them found their way to the black tents and weaver’s workshops, even though large volumes were sold by the industry until WWII and their wet- and light fastnesses were better than those of acid dyes. I have yet to come across a sure identification in the carpet literature. Some may be hidden under the many yellows and oranges which Dr. Mushak could not identify, no doubt for lack of reference-dyes for his HPLC method (but their Cr mordant could have been traced by AES). Today, only black mordant dyes are still in use, but not for carpet yarn (see below, after-chrome dyes).
In 1897, BASF succeeded in manufacturing synthetic indigo, displacing the natural variety from the market. This was a huge and perennial commercial success.
After WWI, synthetic indigo gradually lost its importance as a wool dye (but became a tremendous success as the mandatory dye for cotton denim).
In 1920, Geigy AG and BASF introduced the first range of synthetic dyes specifically developed for wool yarn- and piece dyeing, the levelling acid 1:1 chrome-complex dyes, misleadingly called chrome dyes by most rug weavers. They are small molecules of medium hydrophobicity, mostly from azo chemical family, featuring one chromium III atom permanently bound to the chromophore.
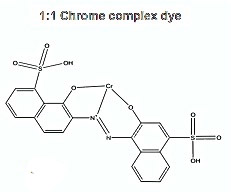
They feature a medium wet fastness, perfectly adequate for rugs, which are not supposed to be warm washed. These dyes do not run if properly applied. They also have very good light-fastness ratings (higher than 4-5 on the Blue Scale) and excellent levelling power. This is especially important for rug yarn, since it must sometimes be dyed in rather rudimentary vessels. 1:1 chrome complex dyes are not the brightest synthetic dyes, but still are mostly somewhat brighter than the mordanted natural dyes and synthetic alizarine, which they displaced from the market. For the first time since mankind found a way to dye wool, a range was made of so-called compatible dyes, suitable for trichromy dyeing. This dyer’s gobbledygook simply means that two or three dyes can be mixed together in the same vessel without problems of shade reproducibility, levelness or fastness.

For example, to make any shade of green, one simply mixes the right proportion of a blue and a yellow. A grey will be made by mixing the right proportion of a yellow, a red and a blue (or mixing an ochre, a navy and a yellow, etc.). This is not possible with natural dyes, which are essentially not compatible and require successive dyeing operations in order to obtain non-corroding black, violet, green or purple. A natural green shade, for example, required first an alkaline vat dyeing with indigo, followed by thorough rinsing and a mordant- acid dyeing with a yellow (weld, for example). The combination of both advantages (slightly brighter shades than their natural counterparts and suitability for trichromy dyeing) has an interesting consequence for 1:1 chrome-complex dyes, not quite fathomed by the average carpetologist: all shades feasible with natural dyes can be perfectly matched and easily reproduced with acid 1:1 chrome complex dyes. All it takes is a very basic understanding of trichromy dyeing principles, accessible to even the most moronic dyer.
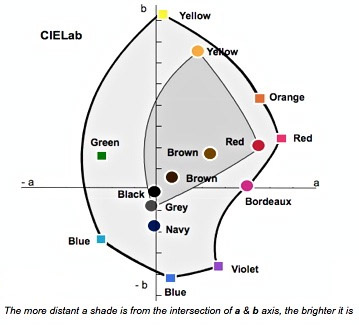
The pale grey area in this figure describes a slice of the CIElab colour domain (shade range) accessible when dyeing with dichromies (mixes of two dyes) or trichromies (mixes of three) of the hypothetical outer dyes, assuming each of those seven dyes can be combined safely with any of the six others (compatible dyes). It is a matter of course that this range can also perfectly match any shade achievable with the three duller yellow, red and grey dye (dark grey area). In this example, the two brown shades could be made both with the brighter trichromy, yellow, red and blue and with the duller one. It is definitely not true that matching natural dyes shades with chrome dyes would come out "metallic” and “cold”, and that a trained eye can identify them.
Of course, since single 1:1 chrome-complex (yellow, orange and red-) dyes are slightly brighter than natural ones, the dyers can create some shades that cannot exactly be matched with natural dyes. However, the difference of brightness which can be achieved with this group of dyes, even intentionally, would remain quite limited and therefore would require a very good eye to be spotted. The next figure shows the shades of the most important 1:1 chrome complex dyes.

1:1 chrome dyes are applied in strongly acidic dye baths (pH 2-2.5), which makes thorough rinsing and neutralising (bringing the internal pH of wool back to its isoelectric point) necessary in order to avoid later fibre degradation. A second generation of these dyes, requiring less acidity (pH 3.5), was introduced around 1970. Today, acid 1:1 chrome complex dyes are by far the most used for hand woven rugs. All other modern synthetic dyes mentioned below are used in other market segments such as tufted rugs (home- or car upholstery) or apparel. I shall therefore not elaborate much on any of these further five groups.
From about 1910 onwards the industry introduced greatly improved ranges of acid dyes, the levelling acid dyes: small, hydrophilic, mono- and di-sulfonated, metal-free molecules of the azo and anthraquinone chemical families. They have low affinity for wool and excellent diffusion in the fibre. Their light fastnesses are better than those of their lousy ancestors (rating 4 or better on Blue Scale). Some very bright dyes are part of the range, even a few that are fluorescent but poorly lightfast. The wet fastness of levelling acid dyes remains rather poor (they still have a tendency to run!); they are not recommended for dyeing rug yarn. Applied to wool from a mildly acidic dye-bath ( pH 5.5), they are mainly used for piece dyeing, usually for pale shades and only where the end-use tolerates their poor wet fastness.
Around 1880, the acid milling dyes started their long, now nearly exhausted career. They are poly-sulfonated, bulky molecules from the azo, anthraquinone and phtalocyanine families; mostly metal-free; more wet fast than acid levelling dyes. Their slow diffusion makes them unsuitable for yarn dyeing. Thus, they are never used for carpet.
The so called after-chrome black dyes are the last survivors of the once large family of synthetic mordant dyes. They are bulky, metal-free dye molecules, similar to milling dyes but can form stable complexes with metals. They are first dyed on wool in a mildly acidic dye-bath, then post-mordanted with chromium ions. This is an inexpensive way to make wet- and light-fast black shades. However, only competent dyers using modern dyeing equipment should be allowed to work with them, in order to avoid formation of toxic and carcinogenic chrome VI in the dye-bath (effluent and textile contamination as well as exposure of dye-house workers are possible). Professional handling guarantees that only comparatively harmless Cr III can be found in the effluents. They are never, to my knowledge, used for dyeing rug yarn.
In 1949, Schetty (Geigy AG) invented the highly successful 1:2 chrome (and cobalt) complex dyes. They are bulky, very hydrophobic molecules with two chromophores forming a complex with one atom of chromium III. This is an extremely stable metal complex. These dyes form rather dull shades. There is no free chromium in the effluents. They have very good wet fastness and very good to excellent light-fastness (rating 6 or better on Blue Scale). Their slow diffusion makes them unsuitable for dyeing wool yarn.
In 1950, Hoechst AG (soon superceded by Ciba AG), created the first reactive wool dyes. These form covalent bonds with keratin fibres, meaning that they become part of the wool chemical structure, unlike all other dyes which are merely absorbed and physically retained inside the fibre. They are mostly metal-free, and are extremely wet fast due to the covalent bond. They show quite good diffusion until the chemical reaction with keratin occurs. Their light fastness is good to very good. Some very bright shades available. They are suitable for wool yarn dyeing, although reactive dyes are never used for hand-woven rugs to my knowledge.
Some information useful for any carpetologist ambitious enough to try to date a carpet based on its colors
a) All shades feasible with natural dyes can be perfectly matched with modern synthetic 1:1 chrome complex wool dyes. There is no way, even for a trained colorist’s eye, to tell the difference if a competent dyer has so decided. The hard, metallic aspect of shades obtained from these synthetic dyes is a myth.
b) The only sure way to tell whether a dye is natural or synthetic is to identify it by chromatography, either using the older and simpler thin layer (TLC) method or the modern high performance liquid method (HPLC), possibly coupled with DAD (diode-array detector) and MS (mass spectroscopy). The latter method requires costly hard- and software and skilled technicians. In both cases, however, the key to proper identification is an extensive collection of pure dye samples that are used as reference. Very few such data banks exist, mainly in the analytical services of two or three large dyestuff manufacturers. This puts dye identification well beyond the reach of even the most suspicious carpet buyer.
c) Modern HPLC/DAD/MS analysis of a blue wool sample can even give an indication as to the type of indigo used (natural or synthetic), since natural indigo contains more colored impurities (indirubin and others) than the synthetic variety. The same is true for natural (from madder) and synthetic alizarin. The method also allows identification of the botanical species from which the natural dyes came.
d) For the sake of completeness, I should mention another analytical method, never used to my knowledge by carpetologists: Plasma Emission Spectroscopy (PES- or AES-) identification of chromium. Any positive test response would give a near certainty that the dye is either synthetic (1:1 chrome dye, post 1920) or a natural dye mordanted with Cr (post-1890), since chromium was hardly ever used with natural dyes before the end of the 19th century. However, a negative result would not guarantee that the dye is natural; it could be a synthetic acid dye, for example. By the same token, a PES identification of aluminum or iron would indicate the use of natural dyes; these mordants and complexing agents have never been used for commercial synthetic dyes. But this would tell us nothing about the date of this dyeing operation, which could be either antique or recent. Identification of copper would give no clue to the type of dyes, Cu having been used both as mordant for natural yellow dyes (to achieve green shades or to improve light fastness) and as a complexing atom in synthetic dyes (copper phthalocyanin turquoise, post 1935).
e) The only other analytical way to date a carpet, C14 analysis, is valuable for dating rugs from the 17th century or older (the older the carpet the more precise it becomes), but it is essentially useless for any carpet less than 150 years old.
As a cynical Italian politician used to say, Always expecting the worst behaviour of our fellow man might be sin, but surely it is the safest assumption. Therefore, points a), b) and e) might explain why so many antique and old carpets are sold as 19th century and so few as 20th. It might also be that most of the large modern production of "natural dyed carpets", riding the recent eco-wave, are in fact dyed with 1:1 chrome dyes (the dyeing method is much simpler, much more reproducible and much cheaper than using natural dyes).
There are a few cases where a synthetic dye can be identified by its too bright shade with some degree of confidence, since the following shades cannot, to my knowledge, be achieved with any usual natural dyes:
a) Bright bluish red, purple and violet: Madder and insect reds cover the shade area from ripe tomato to brick with alum mordant. With careful tin post-mordanting a bright yellowish red (scarlet) is achievable. With alum mordant followed by post-mordanting with traces or iron, madder delivers only dullish purples and violets. With more iron the shade becomes brown. Any bluish red/purple/violet based on double dyeing with indigo followed by madder or insect red will have dullish shades. A very bright bluish red, purple and violet indicates utilization of synthetic dyes of the first generation of acid dyes, like Amaranth, Fast Acid Magenta, Ponceau 6R (post 1870) or of its second generation (post 1910). Aniline dyes like Magenta, Fuchsine or Victoria Blue (a violet dye) led to very bright shades on wool too, but only for a very short time. They quickly turned dull (beige-grey) owing to their lousy light fastness. Please remember that the ubiquitous 1:1 chrome complex red dyes (post 1920) are only slightly brighter, at best, than natural reds.
b) Very bright orange: The occurrence of such a shade probably indicates the use of Orange II, Orange IV, Ponceau RR (a reddish orange) or of one of many similar acid dyes (post 1870). Natural pale-to-medium orange shades are usually obtained using the second or the third spent bath of a madder dyeing batch or, alternatively, by successive dyeing with madder and a yellow. Both deliver subdued shades. One of the brightest natural shade is the warm golden orange achievable with Lawsonia inermis (henna), but its unmordanted direct dye (lawson) lacks lightfastness and was not used on rug yarn, to my knowledge.
c) Very bright turquoise: The best one can do with natural dyes is a subdued turquoise, by double dyeing with indigo and a greenish natural yellow, for example, Reseda lutea mordanted with copper. A bright turquoise indicates the use of either Saxon blue (disulfonated indigo with very poor wet fastness) which can be found on carpet from 1755 onwards, or of a copper-phthalocyanin acid dye, from 1935 onwards
d) Very bright reddish blue (royal blue): In low concentration, Indigo (the only natural blue dye ever used on rugs) delivers a clear, slightly greenish sky blue (the degree of greeness very much depends on the yellowness/ageing of the wool). With repeated indigo dyeing operations, the shade gets progressively deeper, duller and redder, ending with a navy blue. A bright royal blue indicates the use of an anthraquinone acid dye, for example Solway Blue or, more likely, one of its brighter successors, a di-amino anthraquinone (post 1900).
e) Bright and strong green: Subdued almond-green pale shades are achievable with several natural yellow dyes (Reseda lutea, for example), with a careful copper or alum + copper mordanting. They feature good light fastness, but copper is said to corrode wool. A pale-to-medium green featuring a truly lousy wetfastness (blue running) and poor light fastness (pile weaker than back) is probably based upon Saxon blue and a natural yellow. Saturated green natural shades are all based on successive dyeing operations with indigo and a natural yellow (Al or Al+Cu mordant), and are invariably rather dull. These natural green shades are also the ones leading to most abrash. Bright saturated green shades indicate the presence of a synthetic diamino anthraquinone acid dye (post 1900) or of a copper (or nickel) phthalocyanin turquoise acid dye (post 1935).
f) Contrary to frequent assumptions, bright (cold and metallic) lemon yellow-, bright neutral yellow- and bright golden yellow shades are achievable with natural dyes on a bright white wool. The first with Reseda lutea mordanted with alum and then over-mordanted with traces of copper, the second with Datisca cannabina (false hemp, "Gence"), the third with Delphinium zalil (alum mordant). These bright yellow shades are, therefore, not reliable markers for synthetic dyes.
Poor lightfastness is only a sure marker for synthetic dyes in one case: the so-called aniline dyes. These triphenyl methane dyes all share a miserable light fastness on wool and all turn ugly beige or grey very quickly. A beige pile and a mottled bluish red-, violet-, reddish blue- or green back is a pretty sure indicator of this first generation of synthetic dyes (post 1860). True, several other early synthetic dyes also had rather limited light-fastness (Blue Scale ratings 2-3), for example, several orange-, scarlet- and red acid dyes. But these were no worse than several natural yellow dyes mordanted with alum, (natural yellows containing mainly quercetin like dyer’s buckthorn, Alium cepa etc.) or than a natural red quite frequently used in Safavid Persian- or in Ming Chinese carpets (Carthamus tinctorius).
Abrash is not a sure way to tell whether a dye is natural or synthetic
Abrash, meaning small local differences in strength and shade of the rug pile, has 3 main causes with cumulative effects:
1. The heterogeneous structure of wool which explains some local differences of dye uptake on the yarn, even in the same dye bath and on modern dyeing machines.
2. Insufficient dye bath circulation in and around the yarn, which exposes different parts of the fibre to different dye concentrations. This factor is minimized in modern dyeing vessels, where homogeneous bath distribution is warranted by strong pumps and quick rotation of the wool skeins. But it is very important in hand-agitated, primitive dyeing vessels used by nomads, village dyers and, generally speaking, by all pre-industrial dyers.
3. The fact that some dyers (in particular nomads, for obvious logistical reasons) utilize wool from different dyeing operations when weaving a carpet. To obtain exactly the same shade on wool in two successive dyeing operations requires a level of standardization of the wool quality (constant whiteness, fineness, spinning tension, etc.) and of the dyeing operation (pH, temperature profile, duration, etc.) fully out of reach of any small dyer or nomad.
Natural and synthetic dyes will both lead to significant abrash when conditions are unfavorable. It is also quite easy to maximize abrash on purpose and even to make it look natural. It is true, however, that the heterogeneous nature of natural dyes (whose shades depend on many unpredictable factors) significantly increases the risk of abrash. And it is also true that indigo is more prone than any other natural or synthetic dye to levelness (abrash) problems, especially in pale to medium shades, due to its very unstable dye bath behaviour (unwanted early oxidation, with formation of the pigment).
Abrash appearing during ageing of a carpet is, in my humble opinion, at best a minor issue, except perhaps in shades containing a significant indigo component, since a poorly controlled indigo dyeing could lead to randomly distributed, rather large deposits of pigment on the fibre surface. This «surface pigmentation» might be less lightfast than the indigo molecules absorbed inside the fibre.
Running colour (poor wet fastness) hints at early synthetic dyes, but does not give certainty.
Saxon blue and several early acid dyes (especially di-and trisulfonated reds) and most aniline dyes ran when the carpet was kept wet for some time. However, running can occur even with wet-fast natural or synthetic dyes when the following conditions are combined:
1. Insufficient washing of the wool skeins after dyeing. At the end of the dyeing operation only part of the dye molecules are absorbed inside the fibre and bond by physical links to keratin. A significant amount merely sits on the fibre surface or disperses in the wool grease (lanolin) as water-soluble dye or as poorly soluble pigment. This is particularly true when the dye has a low affinity for the fibre (which is the case for all natural and synthetic dyes used for rug wool). This surface dye easily runs when the carpet is wet. Thorough, repeated washings eliminate surface dye.
2. Poor dyeing: Insufficient pre-dyeing scouring of the wool, too low dyeing temperature, too short dyeing- or mordanting time, notable oxidation of indigo early in the dyeing operation, or heavy pre-mordanting all increase the probability that unfixed dye would run.
Running problems are worst in red shades because the eye notices and properly identifies red bleed much more readily than blue bleed (which in very pale shades merely looks whiter) or yellow bleed (which in very pale shades merely looks dirtier).
For the sake of completeness I should add that the nature of the liquid contamination, its temperature and the time of exposure, also influence the amount of bleed. For example, alkaline solutions (ammonia, urine, etc.) reduce the wool affinity of dyes, which obviously favors bleed.
Is pile corrosion a sure marker for dyeing recipes using natural dyes (like black- and brown shades based upon tannin and iron salt, green shades based on natural yellows and copper mordant, etc.)?
I have no clear opinion, for lack of experience in this area, but would make the reader aware that several dyer’s mistakes with synthetic dyes could also lead eventually to pile corrosion. For example, dyeing with 1:1 Cr complex dyes is always performed at a very low pH (2.0-3.5), and irreversible wool degradation could indeed be caused:
1. by keeping the dyebath at 100°C for too long (say, 3-4 hours instead of the usual 45-90 minutes),
2. by dropping the pH even lower than absolutely necessary (error in dosing the sulfuric acid),
3. by insufficient rinsing and buffering of the wool skeins immediately after dyeing The rinsing is necessary to bring the internal pH of wool near to its safe isoelectric point. I owned a small Afghan rug (recent, thus probably dyed with synthetic dyes) with very brittle red wool pile which actually disappeared in the vacuum cleaner in less than three years!
I hope that this little essay will be useful for some fellow carpetologists.
Pierre Galafassi